Abstract
Introduction: Pre-existing adeno-associated virus (AAV) neutralizing antibodies (NAbs) have limited the efficacy of AAV-based gene therapy in prior clinical applications, including in hemophilia. A unique aspect of the Phase 3 HOPE-B clinical trial (NCT03569891) assessing etranacogene dezaparvovec gene therapy, an AAV serotype 5 vector expressing Padua factor IX (FIX), was the enrollment of participants regardless of their baseline AAV5 NAb status. The HOPE-B clinical trial met its primary efficacy endpoint, providing hemostatic protection superior to standard of care FIX prophylaxis over 52 weeks of follow-up after stable FIX Padua expression (defined as Months 7-18).
Aim: Assess efficacy and safety of etranacogene dezaparvovec in HOPE-B participants with (NAb+) and without (NAb-) pre-existing AAV5 NAbs over 18 and 24 months of follow-up.
Methods: Adult male participants with severe or moderately severe hemophilia B (FIX ≤2%), NAb+ or NAb-, were treated in the Phase 3, open-label, single-arm, HOPE-B trial with a single intravenous infusion of etranacogene dezaparvovec (2x1013 gc/kg), following a ≥6-month lead-in period receiving FIX prophylaxis. FIX activity, annualized bleed rate (ABR), and use of infused replacement FIX concentrates were assessed regularly during the lead-in and the first 12 months after receiving etranacogene dezaparvovec, then every 6 months during the long-term follow-up (Years 2-5). Adverse events were recorded continuously. Although not an exclusion criterion, AAV5 NAbs were assessed on the day of intravenous dosing (baseline), using a custom-developed, cell-based in vitro AAV5 transduction inhibition assay (sensitivity 10 ng/mL antibody, titer 1:7, intra and inter-run coefficient of variation [CV] <30%).
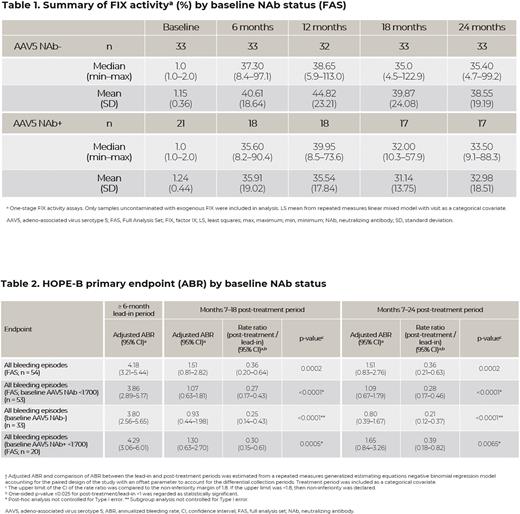
Results: Of the 54 participants who received etranacogene dezaparvovec, at baseline 33 participants were NAb- and 21 were NAb+. The median (Q1-Q3) titer among NAb+ participants was 56.9 (23.3-198.9) and 20/21 (95%) NAb+ participants had titers of <1:700. One participant with a markedly high NAb titer of 3212 prior to vector dosing and one participant who only received a partial dose (due to an infusion-related reaction; NAb titer: 198.9), did not express FIX Padua and did not discontinue FIX prophylaxis. All other participants (52/54) discontinued FIX prophylaxis.
At 18- and 24-months post-dose, no clinically meaningful correlation between an individual's baseline AAV5 NAb titer and FIX activity levels was identified, up to a NAb titer of <1:700 (24-month Pearson coefficient: -0.29; Spearman coefficient: -0.25; R2: 0.086). Sustained FIX activity levels (median [min-max]) were demonstrated at 18 months post-dose in NAb+ <1:700 (32.0% [10.3-57.9]) and NAb- (35.0% [4.5-122.9]) participants and at 24 months post-dose in NAb+ <1:700 (33.5% [9.1-88.3]) and the NAb- (35.4% [4.7-99.2]) participants (Table 1).
At both 18- and 24-months post-dose, NAb+ <1:700 and NAb- participants demonstrated a low ABR (Table 2). The ABR achieved by the NAb+ <1:700 and NAb- subgroups at 18 months was 1.30 and 0.93, and at 24 months was 1.65 and 0.80, respectively. These ABRs were significantly improved compared with the respective ABRs of 4.29 (p=0.0005 and p=0.0065 at 18 and 24 months, respectively) and 3.80 (p<0.0001 at 18 and 24 months) observed during the ≥6-month lead-in period of continuous FIX prophylaxis.
The safety profile of etranacogene dezaparvovec was similar between NAb subgroups. At 24 months, corticosteroid-treated transaminase elevations occurred in 6/33 NAb- (18.2%) and 3/21 NAb+ (14.3%) participants. Infusion-related reactions occurred in 2/33 NAb- (6.1%) and 5/21 NAb+ (23.8%) participants. There was no statistically significant association between infusion-related reactions and NAb status (p=0.0956).
Conclusions: Throughout 24 months of follow-up, AAV5 NAb- and NAb+ (<700 titer) HOPE-B participants receiving etranacogene dezaparvovec demonstrated significant reductions in ABR, freedom from continuous FIX prophylaxis and a comparable and acceptable safety profile, regardless of NAb status. FIX activity levels were stable, with no association between baseline NAb status (up to titer <1:700) and the long-term durability of FIX expression.
Disclosures
Pipe:Pfizer: Consultancy; Novo Nordisk: Consultancy; Roche/Genentech: Consultancy; Sangamo Therapeutics: Consultancy; Freeline: Consultancy; HEMA Biologics: Consultancy; CSL Behring: Consultancy; BioMarin Pharmaceutical Inc.: Consultancy; Bayer: Consultancy; ASC Therapeutics: Consultancy; Apcintex: Consultancy; Sanofi: Consultancy; Takeda: Consultancy; Spark Therapeutics: Consultancy; UniQure: Consultancy; Regeneron/Intellia: Consultancy. Leebeek:Biomarin: Consultancy; uniQure: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; CSL Behring: Research Funding; Sobi: Consultancy, Honoraria, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees. Recht:Oregon Health & Science University: Ended employment in the past 24 months; Foundation for Women and Girls with Blood Disorders; Partners in Bleeding Disorders: Thrombosis and Hemostasis Societies of North America: Membership on an entity's Board of Directors or advisory committees; Bayer, Biomarin, CSL Behring, Genentech, Grifols, Hema Biologics, LFB, Novo Nordisk, Octapharma, Pfizer, Sanofi, Spark Therapeutics, Takeda, uniQure: Research Funding; Catalyst Biosciences, CSL Behring, Genentech, Grifols, Hema Biologics, Novo Nordisk, Pfizer, Sanofi, Takeda, uniQure: Consultancy; American Thrombosis and Hemostasis Network; Yale University School of Medicine: Current Employment. Key:Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; uniQure / CSL Behring: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Biomarin: Membership on an entity's Board of Directors or advisory committees. Lattimore:uniQure: Honoraria, Membership on an entity's Board of Directors or advisory committees. Castaman:Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Kedrion: Consultancy, Honoraria, Speakers Bureau; Werfen: Consultancy, Honoraria, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CSL Behring: Membership on an entity's Board of Directors or advisory committees, Research Funding; Grifols: Consultancy, Honoraria, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Ablynx: Membership on an entity's Board of Directors or advisory committees; Alexion: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; uniQure: Membership on an entity's Board of Directors or advisory committees. Cooper:uniQure: Current Employment. Verweij:uniQure: Current Employment. Dolmetsch:uniQure: Current Employment. Tarrant:CSL Behring: Current Employment. Li:CSL Behring: Current Employment. Monahan:CSL Behring: Current Employment. Miesbach:Freeline: Consultancy; Pfizer: Consultancy; LFB: Consultancy; Roche: Consultancy; Takeda: Consultancy; Biomarin: Consultancy; Sobi: Consultancy; uniQure: Consultancy; Novo Nordisk: Consultancy; Chugai: Consultancy; Biogen: Consultancy; Alnylam: Consultancy; Bayer: Consultancy; Octapharma: Consultancy; CSL Behring: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.